
By: Rosli Mansor
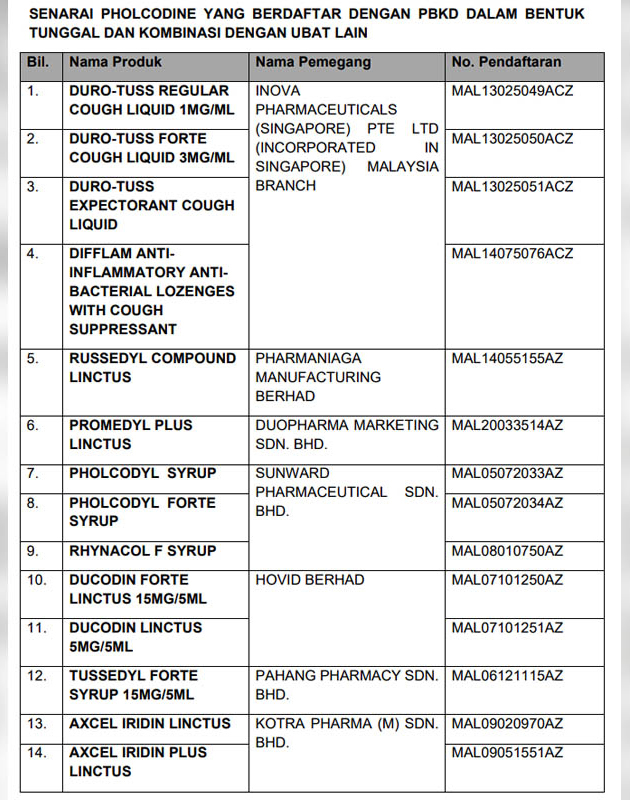
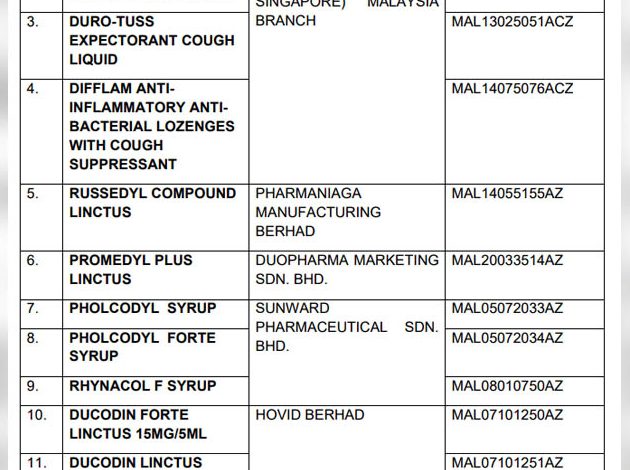
KUALA LUMPUR – The Ministry of Health’s Drug Control Authority (PBKD) has agreed to cancel the registration and issue a recall order on all registered products containing pholcodine.
According to the Malaysian Ministry of Health (MOH) yesterday, people who have taken drugs containing pholcodine (usually cough medicine) in the past 12 months face a higher risk of experiencing anaphylaxis ( a severe allergic reaction that could lead to death) if given muscle relaxants or neuromuscular blocking agents (NMBAs) during the general anaesthesia process, for example during surgery,” said the statement.
Pholcodine is a medicine to control and stop dry cough (nonproductive/dry, irritating) in adults and children.
In Malaysia, pholcodine is classified as a Group C controlled drug and can be obtained without a prescription.
All remaining stock of products containing pholcodine should be quarantined and returned to the supplier.
The general public who are taking cough and cold medicine are reminded to check the label or insert of the product package, whether there is the active ingredient pholcodine. If it is found that the medicine contains the active ingredient pholcodine, stop taking it and refer to a health professional for alternative treatment.
For those who have to undergo surgery and require full anaesthesia, inform the health personnel if you have taken a medicine containing pholcodine, especially in the past 12 months.